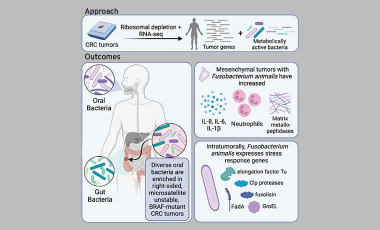
In parellel to studying the microbiome in patients with cancer or autoimmune diseases, the Byrd lab studies samples from healthy volunteers to understand factors influencing gut microbiome and metabolome composition in the steady state. To accomplish this, we collaborated with the Institut Pasteur’s academic consortia, Milieu Intérieur, to build a robust microbiome dataset of 1,359 gut microbiome samples from 946 healthy donors (Byrd et al JEM 2021). Using a novel reference database, we found biological sex and age were the strongest drivers of community composition, meaning these variables must be considered when running microbiome statistical analyzes. Accounting for these factors, we defined global shifts in the microbiome of patients with non-gastrointestinal tumors compared with healthy donors. While microbial dysbiosis had been well described for patients with colorectal cancer this was the first-time patients with non-GI cancers had been identified to have shifted microbial communities compared to age-matched healthy donors. More specifically, we found cancer patient’s gut microbiomes had decreased bacterial diversity and increased abundances of Bacteroides species. Moving forward, we will continue to leverage this healthy dataset as a baseline to which disease cohorts will be compared.
To complement these taxonomic-based findings, the next stage of the collaboration is focused on metabolomics. Because gut microbes have unique capabilities to derive and modify many metabolites, we hypothesize that microbially influenced metabolites are an important mechanism by which microbes modulate host immune activation and tissue physiology. As such, dysbiotic microbiomes, common in individuals with myriad autoimmune diseases, may contribute to aberrant immune responses via altered metabolomic profiles.
Additional Research Projects
Longitudinal microbiome dynamics in patients with cancer and immune-mediated diseases