Plasmacytoid dendritic cells (pDCs) are innate immune cells specialized for responding to internalized nucleic acids via endosomally-localized TLR7 and TLR9, resulting in potent IFNα production. During viral infection, IFNα production by pDCs is protective. However, pDC production of IFNα is pathogenic in the autoimmune disease Systemic Lupus Erythematosus (SLE), where IFNα and signatures of this cytokine family are elevated and correlate with disease activity and severity. SLE is also characterized by a loss of tolerance to nuclear antigens, resulting in the production of circulating autoantibodies to DNA, RNA, and nucleic acid binding proteins. These autoantibodies form immune complexes with nuclear antigens released from dying cells, which can be internalized by immune cells expressing receptors for the Fc portions of the autoantibodies in the complexes (FcRs). pDCs internalization of immune complexes in SLE leads to potent IFNα secretion, and this IFNα amplifies disease through its pleiotropic effects on the immune response, including increasing plasmablast differentiation and antibody production, leading to a feed-forward loop between B cells, antibodies, and pDCs that exacerbates disease.
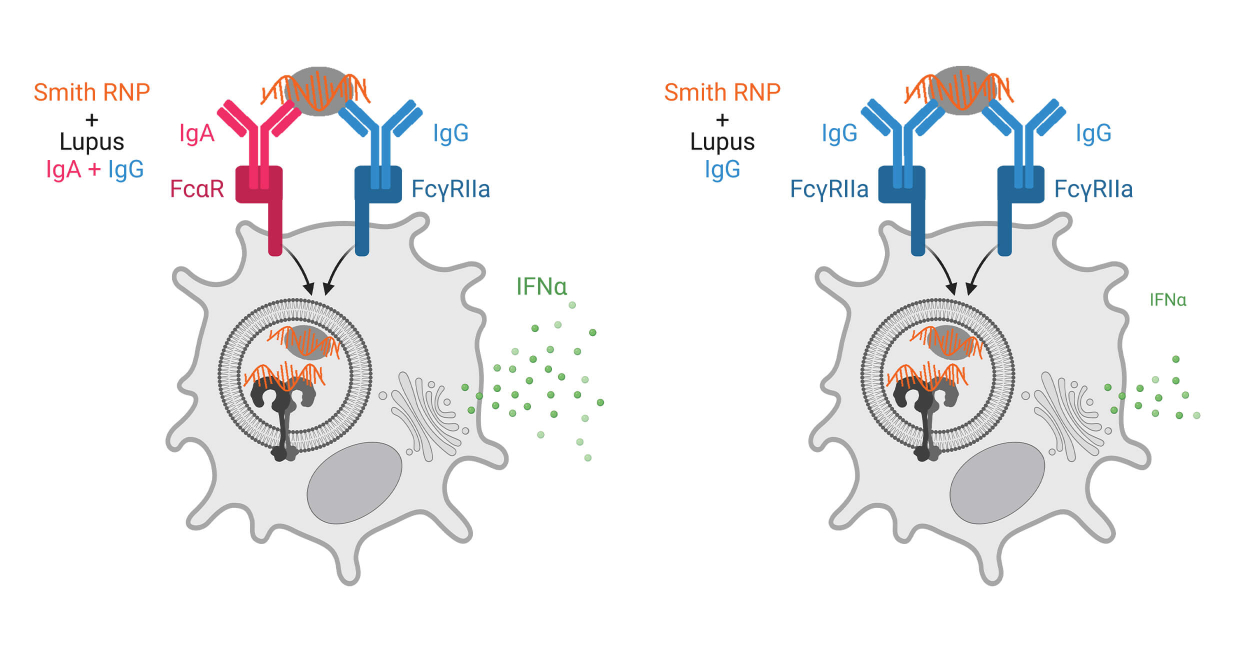
Most studies of autoantibodies in SLE focus on IgG antibodies as this is the major circulating antibody isotype. Additionally, pDC IFNα secretion in response to SLE immune complexes depends upon recognition of IgG antibodies by FcgRIIa (CD32a). However, anti-nuclear antibodies (ANAs) of other isotypes, such as IgA and IgE have been documented but not well studied. Our work has focused on IgA autoantibodies in SLE. We have found a previously unrecognized role of IgA and its receptor FcαR (CD89) on pDCs in recognizing immune complexes made with autoantibodies from individuals with SLE when complexed with RNA-containing Smith ribonucleoproteins.
Our work shows a potent synergy between IgG and IgA autoantibodies allowing for more efficient immune complex binding and internalization and subsequent IFNα secretion by pDCs. In conjunction with these findings, we also found that pDCs from SLE donors had greater immune complex internalization than those from control donors, that this internalization correlated with surface FcαR expression, and that pDCs from SLE donors expressed more FcαR than from control donors. Together, these findings suggest that immune complexes containing both IgA and IgG are important in driving SLE disease. We are currently investigating the mechanisms for synergy between FcαR and FcgRIIa, in pDCs, the regulation of FcαR expression on pDCs in SLE, and defining the spectrum of IgA autoantibody specificities in SLE and how they are associated with disease manifestations.
Additional Research Projects

Flightless-1 in lung macrophage and DC development and function