In myeloid cells, the actin cytoskeleton controls important functions including adhesion, migration, and phagocytosis. Actin capping proteins both positively and negatively regulate the actin cytoskeleton, with both unique and overlapping functions.
We have a particular interest in Flightless-1, a member of the gelsolin family of actin capping proteins that has principally been studied in non-hematopoietic cells. In macrophages cells, Flightless-1 is a negative regulator of the NLRP3 and NLRC4 inflammasomes, which we showed depends upon the signaling adaptor BCAP. To investigate the role of Flightless-1 in myeloid cells in vivo, we generated a conditional knockout allele and deleted in either monocytes, macrophages, and neutrophils or in dendritic cells (DCs), with a goal to investigate how Flightless-1 regulates key actin-dependent processes in these cells. For these studies, we focused on macrophages and conventional DCs (cDCs) in the lung, which are situated to respond to respiratory pathogens.
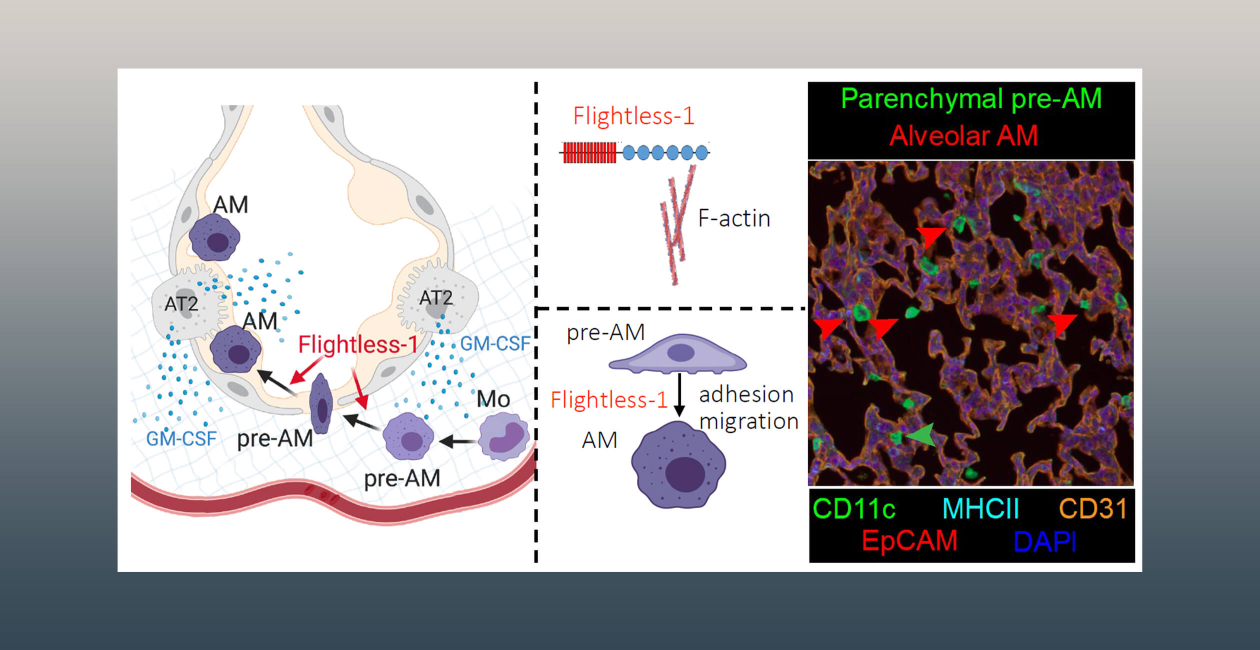
In the lung, alveolar macrophages (AMs) are uniquely situated in the alveoli and are the only tissue macrophage found outside the body’s epithelial barrier. AMs have both homeostatic and immune functions—to keep alveoli clear of debris to facilitate gas exchange and to phagocytose pathogens and initiate inflammation. Underscoring the importance of AMs, deficiency in these cells causes pulmonary alveolar proteinosis (PAP), a disease where the alveoli fill with surfactant and cellular debris causing impaired breathing, and, in severe cases, progressive respiratory failure.
AMs differentiate from fetal monocytes during a short perinatal window supported by the cytokine GM-CSF. During this process monocytes become pre-AMs in the lung parenchyma and then cross the alveolar epithelium to finish their maturation in the alveoli. AM differentiation requires cell adhesion and migration for AM progenitors to become juxtaposed to the alveolar epithelium to receive the appropriate GM-CSF signals for differentiation and to perform the difficult task of crossing the epithelial barrier. We find that deletion of Flightless-1 in monocytes causes a block in AM differentiation with pre-AMs accumulating in the lung parenchyma and unable to cross into the alveoli. GM-CSF upregulates Flightless-1 expression in Ly6Chi monocytes, and Flightless-1 deficiency in these cells causes changes in cell size and shape and the actin cytoskeleton. Therefore, we have uncovered a new role for this actin capping protein in alveolar macrophage differentiation and identified an important function of GM-CSF in regulating the actin cytoskeleton in monocytes through Flightless-1. We are currently investigating precisely how Flightless-1 regulates monocyte and pre-AM adhesion and migration, as well as how it regulates mature AM function and other tissue macrophage populations.
cDCs in the lung are critical for detecting and internalizing pathogens in the airways and then migrating to the draining lymph nodes where they present antigens to T cells. The actin cytoskeleton can regulate all of these key processes. We found that phagocytosis of bacteria and fungal particles in vitro was defective in the absence of Flightless-1. In vivo, deletion of Flightless-1 in cDCs resulted in reduced phagocytosis by cDC1s in the lung and reduced migration to the draining mediastinal lymph node. We additionally found improper positioning of cDC1s further away from airways in lungs from mice lacking Flightless-1 compared to controls in the steady state. Thus, Flightless-1 regulation of actin dynamics is critical in regulating lung cDC1 positioning, phagocytosis, and migration. We are continuing to investigate how Flightless-1 controls cDC function in the lung and other tissues.
Additional Research Projects

Immune complex activation of pDC IFNα production in lupus