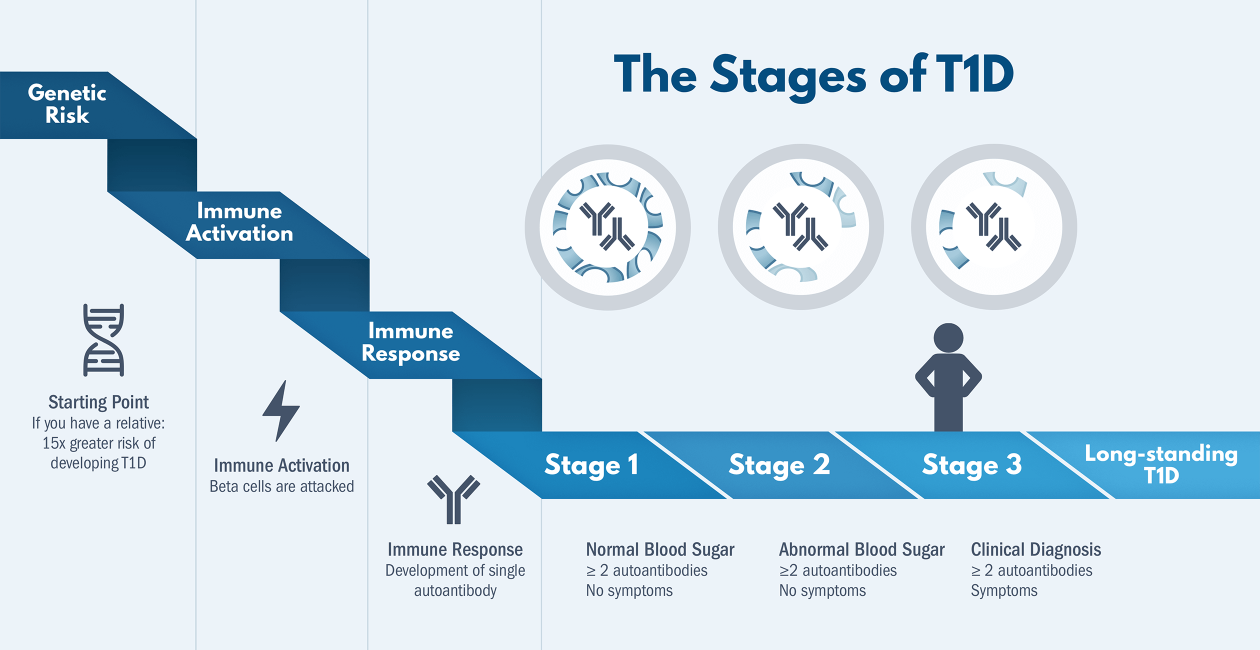
Type 1 Diabetes TrialNet has enrolled people at risk for T1D for over 20 years, and has collected samples and clinical data from those individuals as they progress toward clinical diagnosis. This provides a rich dataset that we have used to ask questions about disease progression and how it differs between autoantibody positive at-risk people.
For example, we found that there is a small group of individuals who lose autoantibodies, transitioning from multiple antibody positive high-risk status to a lower single or zero antibody status – and we show that transition was accompanied by a reduced risk of clinical diagnosis. We are eager to identify immunological mechanisms that may explain this change.
We have also used this dataset to show that some features that predict differing rates of progression vary with age, implying a need to apply statistical methods that are appropriate for age-varying predictors.
An important consideration now that there is a therapy to delay T1D onset is the development and implementation of autoantibody screening and glucose monitoring methods that are accessible to patients through our health systems.
One aim of monitoring is to reduce the number of individuals diagnosed in diabetic ketoacidosis, a life-threatening complication of T1D that occurs much less frequently in people who participate in research studies. In the research setting, study participants typically are seen every 6 months to look for changes in glucose tolerance. We used TrialNet data and novel statistical modeling techniques to show that monitoring for changes in glucose tolerance could be done much less regularly in clinic – but still achieve similar results in terms of prevention of diabetic ketoacidosis.
Additional Research Projects

Rigor and Reproducibility in T1D Biomarker Research